European Medicines Agency (EMA) on Monday says it has approved a new coronavirus vaccine from U.S. manufacturer — Novavax.
The medicines agency, after a special meeting in Amsterdam said the vaccine met the EMA’s standards for effectiveness, safety and quality.
The vaccine is designed to be taken in two doses, about three weeks apart, and has demonstrated 90 per cent efficacy
against COVID-19.
However, it is not clear how powerful the protection it offers is from the Omicron variant, currently spreading worldwide.
Once the European Commission has given its formal approval, Novavax will be the fifth vaccine against COVID-19 to be approved for use in the European Union.
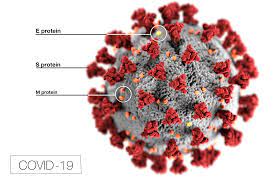
In contrast to the four other shots already approved, Novavax is a protein-based vaccine.
It contains tiny particles consisting of a laboratory-produced version of the spike protein of Sars-CoV-2, the virus that causes the COVID-19 disease.
The Pfizer/BioNTech and Moderna preparations are messenger RNA vaccines while AstraZeneca and Johnson & Johnson’s are vector-based.
This difference may help convince some vaccine sceptics who do not wish to take the vaccines currently on offer in the EU.
Maryland-based Novavax applied for authorization in the EU in November.